The Science & Safety of Carbon Monoxide in
Organ Transplant
A wide range of both preclinical and clinical studies have already demonstrated the safety and efficacy of CO at levels projected to be highly therapeutic in humans. Furthermore, Proterris has developed proprietary dosing and monitoring methodologies, devices and software to further ensure safety, deliver precise CO doses and maximize efficacy. Furthermore, this CO gas-device-dosing-software methodology is designed to be fully utilizable in operating room, intensive care and spontaneously breathing clinical settings.
Amongst the many therapeutic areas and clinical indications addressable with CO therapy, we at Proterris are initially focused on organ transplant, as shown in our pipeline chart below:
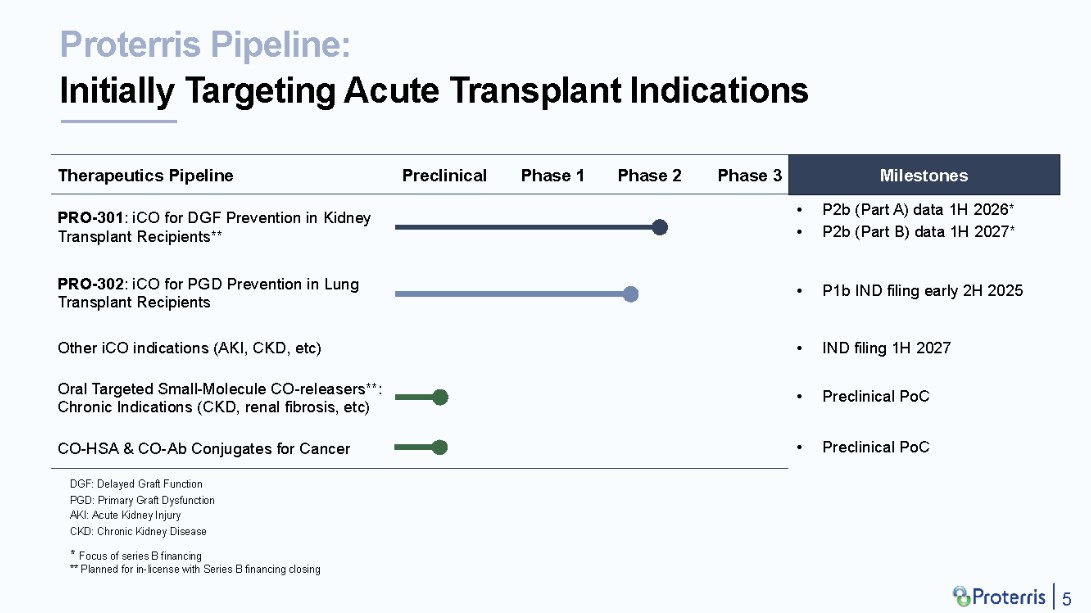
Prevention of delayed graft function (“DGF”) in kidney transplant recipients and prevention of primary graft dysfunction (“PGD”) in lung transplant recipients are both areas of critically unmet need for which there are no approved therapies, significant acute and chronic sequelae and a paucity of candidates in the pipeline. Both conditions are caused by ischemia reperfusion injury (“IRI”) through multi-modal pathologic mechanisms for which a therapeutic with a multi-modal mechanism-of-action (such as CO) is needed to reduce their occurence, optimize organ and patient outcomes (including reducing mortality rates) and reduce healthcare costs.