Safe
CO dosed in over a dozen human clinical studies to date, with tight control over blood CO levels and no major side effects
The Science & Safety of Carbon Monoxide in
Organ Transplant
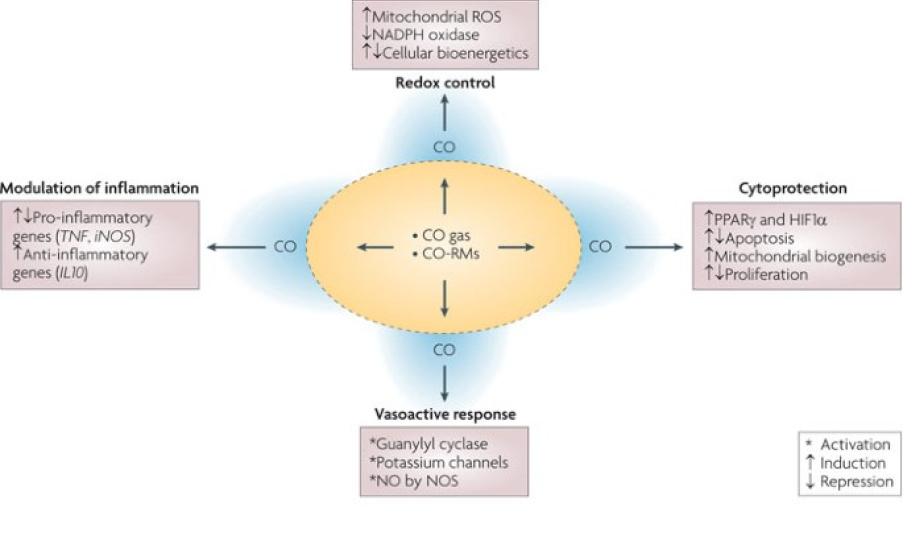
Carbon monoxide (“CO”) has long been recognized and studied for its therapeutic potential given its unique and potent cytoprotective, anti-inflammatory and anti-fibrotic effects. Indeed, amongst the four therapeutic, so-called “gasotransmitter” gases (CO, nitric oxide, hydrogen sulfide and xenon), CO dwarfs the others in terms of preclinical validation in a wide variety of therapeutic areas. In addition, these therapeutic effects are realized at doses far less than what is already firmly established as toxic levels.
To harness this broad potential, Proterris has amassed a broad array of inhaled & small molecule CO therapeutics for transplant, nephrology, pulmonary and oncology applications, which together address a treatable patient population of more than 20 million patients in the U.S. alone.

Controlled CO-dosing has demonstrated strong cyto-protective, immuno-modulatory, anti-fibrotic and anti-inflammatory effects while demonstrating strong safety:
CO dosed in over a dozen human clinical studies to date, with tight control over blood CO levels and no major side effects
>20 studies published in preclinical transplant models, shown to
 Improve kidney and liver preservation in rats and pigs
Improve kidney and liver preservation in rats and pigs Prevent lung ischemia-reperfusion injury and chronic rejection in rodents
Prevent lung ischemia-reperfusion injury and chronic rejection in rodents Improve islet cell survival
Improve islet cell survival  Reduce small bowel transplant IRI in rats
Reduce small bowel transplant IRI in rats Improve cardiac allograft survival in rodents
Improve cardiac allograft survival in rodents Prevent aortic allograft vasculopathy in mice
Prevent aortic allograft vasculopathy in mice